METHOD
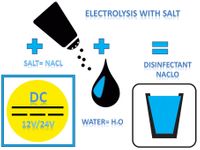
The Cleanaqua process for drinking water treatment and preservation is a patented chamber cell electrolysis using solar energy and commercially available table salt. During this electrolysis, two electrodes connected to a voltage source conduct a direct electrical current into the water.
Important for the electrolysis is the electrical conductivity of the water. Pure (ie distilled or demineralized) water has an extremely low conductivity. The salt present in the water is a good electrolyte and is used in the Cleanaqua process to produce the disinfectant sodium hypochlorite (NaClO), which had been tried and tested in medicine for decades, from water (H2O) and salt (NaCl) by ionization or a so-called "wet oxidation".
The very stubborn biofilm, in which bacteria and viruses are stored in a very short time, is effectively destroyed by the Cleanaqua process, in particular by the high oxygen content (O) produced in the water.Since the salt content varies from water to water, it may be necessary to add common salt to the water to increase the conductivity of the water.
Sodium hypochlorite (NaClO) is listed by the Federal Health Office in the German Drinking Water Ordinance (TVO) and approved as a disinfectant for the disinfection and sterilisation of biologically contaminated drinking water. It should not to be confused with highly concentrated sodium hypochlorite and is produced with Cleanqua appliances in a concentration of 0.2 mg / l, in accordance with the German Drinking Water Ordinance, from natural common salt.